Stanford researchers make ammonia from air and water microdroplets
Green Car Congress
MAY 1, 2023
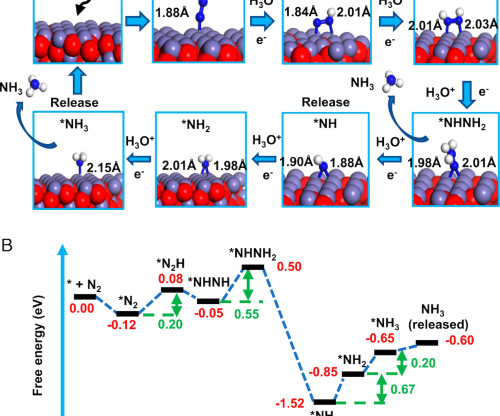
Stanford researchers, with a colleague from King Fahd University of Petroleum and Minerals, have developed a simple and environmentally sound way to make ammonia with tiny droplets of water and nitrogen from the air. Water microdroplets are the hydrogen source for N 2 in contact with Fe 3 O 4. The conversion rate reaches 32.9 ± 1.38





























Let's personalize your content