Is there a better way for scientists to shine a light on nerve cells throughout the body? When researchers Xinyue Liu and Siyuan Rao first began their collaboration at MIT, they treated this question literally.
Optogenetics is an interdisciplinary branch of science in which cells are genetically altered to be light-sensitive, making it possible to inhibit or excite cells and to study their function by applying colored light. Typically, the light-transmitting wires that reach target cells are made of materials that work well when stationary in the brain. However, if they’re implanted elsewhere in a test animal’s body, they could break, damage tissue, or affect behavior, making it difficult to study the peripheral nervous system and pain in particular.
“This flexible fiber expands the toolbox of approaches we have.” —Rob Bonin, University of Toronto
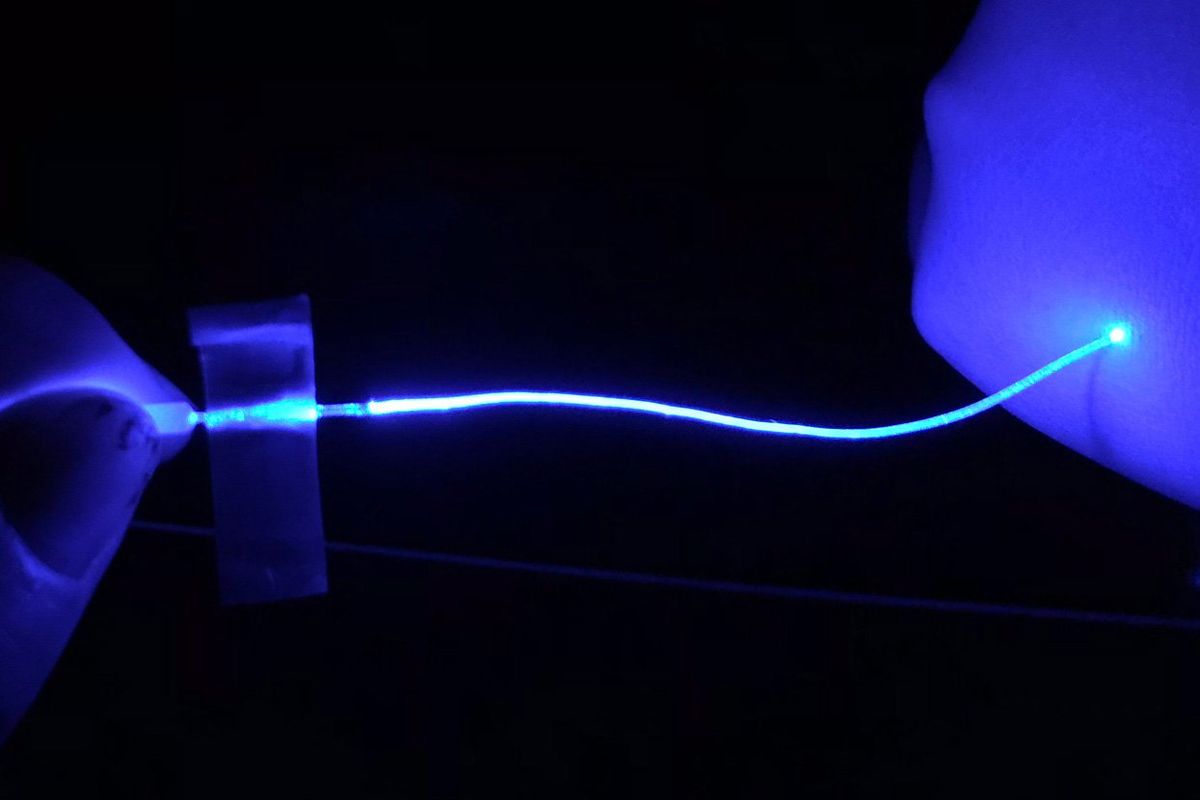
Now, Liu, Rao, and colleagues have developed a soft, flexible, durable optical fiber capable of delivering an optogenetic light signal away from the brain or spine using a new material: hydrogel. The filament consists of an inner core and an outer cladding of two versions of the hydrogel with different refractive properties, yet the fiber is only about one millimeter in diameter.
Researchers described the optical fiber and a variety of ways it was put through its paces in model mice in a paper in Nature Methods, published 19 October. The work adds another technique—and a bit of flexibility—to the repertoire of optogenetics, the study of the peripheral nervous system, and possibly future translational medicine, including the treatment of pain, chronic pain, and nerve disorders.
“This flexible fiber expands the toolbox of approaches we have for peripheral optogenetic work,” said Rob Bonin, a pain researcher at the University of Toronto who was not involved in the research, citing flexibility and durability as two major advantages of the new approach.
Broadly, hydrogels are soft networks of polymers and water, such as tofu or jelly. “Our body is also made of hydrogels. Except for bones and teeth, our muscles and other organs are all actually hydrogels,” said Liu, a materials scientist now at Michigan State University. The fiber uses a polyvinyl alcohol hydrogel, selected for its combination of optical properties and durability under repeated mechanical stress.
The investigation of soft materials was initiated with optogenetic pain research in the peripheral nervous system specifically in mind. “If your implant itself is causing pain, how are you going to use this technology to study pain?” said Rao, a neuroscientist now at the University of Massachusetts Amherst.
And although at the moment the hydrogel fiber primarily figures as a research tool in mice, the same qualities that set this new technology apart for basic science—it’s durable and apparently comfortable in a freely moving body with no compromises in optical performance—are also positives for potential therapeutic purposes. “We are working towards that direction,” said Rao.
The technology promises a wide range of potential applications beyond just the brain and spine.
Researchers anchored one end of their fiber to the mice’s skulls, threaded it underneath the skin, and wrapped a cuff at the other end around the sciatic nerve in the leg. From a practical standpoint, this made the implant compatible with existing external light sources, and kept mice from scratching at any element of the device. But it also worked as a demonstration that enabled a full range of motion of the subject. At a mouse scale, the fiber needed to be only 6 centimeters long, but the authors said it could be extended for other uses.
A series of tests showed that the fiber transmitted light and also how it performed in the mice, blocking pain caused by a hot plate on the foot and inducing movement in the leg. Critically, the fiber performed well after several weeks of voluntary exercise-wheel use, which researchers estimated added up to thousands of bends and twists.
Other optogenetic studies of the peripheral nervous system in mice have attempted various methods of light delivery that don’t use an optical fiber at all, instead shining light through the skin or implanting miniaturized remote devices. In comparison, the new hydrogel fiber should be able to more precisely target specific cells, said Rao.
For Bonin, the external light source has its pros and cons, including higher intensity light and the possibility that a tether could affect behavior, respectively.
Federico Iseppon, a pain researcher at University College London who was not involved in the study, said that although the fiber may be relatively easy to use, it will still require specialized knowledge to fabricate and surgically implant. It promises a wide range of potential applications beyond just the brain and spine. “Its plasticity lies in the multiple different tissues that could be targeted with this technology,” he said.
Liu is currently working on an interface, such as a patch, between the hydrogel and organs that would enable connections that the current cuff design doesn’t allow. Ideally, the fiber will eventually also let scientists record activity as well as send signals to cells.
- With a Better Optogenetic Light Switch, Scientists Can Flip Neurons On and Off ›
- One-Step Optogenetics for Hacking the Nervous System ›
Greg Uyeno is a freelance science journalist based in New York City. He has been an editor, copyeditor, reporter and writer for a wide range of mediums, ages and audiences.