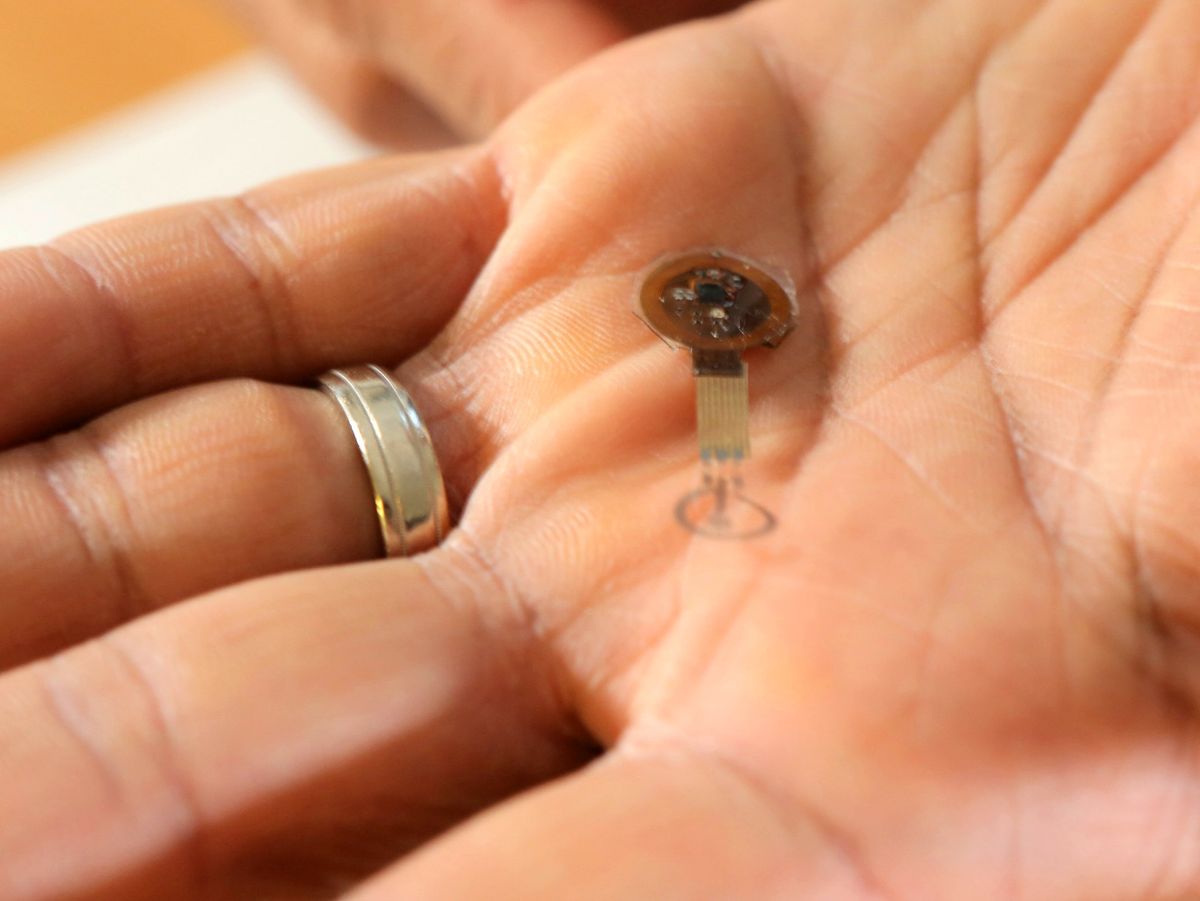
Researchers at Northwestern University, Evanston, Ill., and the University of Sussex, Brighton, England, have created prototypes of new environmentally sustainable devices that can monitor blood pressure and heartbeat, or heal persistent afflictions such as diabetic ulcers.
The devices are also far more advanced than proof-of-concept stage; the Northwestern device, a transient bandage that uses electrotherapy to both monitor and heal diabetic wounds, is resorbed into the body. It may be ready for human trials within a year to 18 months, according to Guillermo Ameer, director of Northwestern’s Center for Advanced Regenerative Engineering. The bandage consists of two small molybdenum electrodes connected to a battery-free power-harvesting unit and a near-field communications module that communicates with control software in a smartphone or tablet.
In a study conducted on diabetic mice published in Science Advances, Ameer and his collaborators, including resorbable electronics pioneer John Rogers, found the device led to 30 percent faster healing than a control group using ordinary bandages.
The device works by transmitting a small current from the outer ringlike electrode, which sits around the wound site, to the inner flower-shaped electrode, which is about 120 micrometers across and sits atop the wound. (The mouse study used about 1 volt of current, and Ameer said that may change in upcoming studies on larger animals.) The current stimulates healthy skin regeneration, the progress of which is measured by current differential between the electrodes. As the wound heals and dries, the current differential decreases.
Perhaps the most compelling element of the device is the inner electrode. As the wound heals, the regenerated skin grows over the electrode and completely absorbs it. The outer ring electrode and the accompanying power and communications unit are detachable from the inner electrode. Results of the mouse study showed molybdenum concentrations in the body returned to those similar to the control group’s within 22 weeks.
Ameer said he and his colleagues would not have moved forward with the idea if they did not think it was safe.
“It’s a matter of risk/benefit, like any other drug or medical device,” he said. “This is not designed for your kid who gets a scratch on their leg. It’s designed for wounds that haven’t healed in a month or so and are predisposed to infection, which leads to complications and amputation. After healing, the skin will grow over the remaining electrode, which you expect over time will be resorbed. You can explain that to the patient and they and their doctor can make an informed decision.”
He said the technology could be particularly useful in parts of the body that are hard to reach or see, such as the bottom of feet. Given that one of the symptoms of diabetes is peripheral nerve damage, Ameer said a patient might be suffering from a wound that is getting worse but not be able to see or feel it. The constant flow of data available from the device to a connected clinician’s dashboard can reduce or eliminate that risk.
A Biodegradable Algae-Based Sensor
While a portion of the Northwestern device bioresorbs itself into the body, the sensor developed at the University of Sussex is completely biodegradable. It is composed of food-grade algae powder added to a graphene suspension composed of graphite, sodium cholate, and deionized water, then dried to form a nanocomposite sheet. When soaked in a yet another food-grade component —a calcium chloride water bath—the sheet swells and creates a conductive hydrogel.

The device, described in ACS Sustainable Chemistry & Engineering, is also extremely flexible for a nanocomposite (with a Young’s modulus just 0.6 pascal), and sensitive enough to measure an object of just 2 milligrams of mass, which the inventors likened to the pressure created by a single raindrop, on its surface. The researchers surmise that the inherent repulsion between the highly hydrophobic and conductive graphene and the hydrophilic but insulating algae gel—which they called “poor interfacial adhesion”—lend it its enhanced sensitivity to mechanical deformation. “In terms of application as mechanical strain-sensing devices, these low mechanical properties are highly advantageous as they give rise to an exceeding low onset deformation for electromechanical response,” they concluded.
For initial applications, they envision the gels could be used as environmental sensors in a range of applications including rainfall detection and sensing of air-current leakage for more efficient heating or cooling of buildings.
The study’s corresponding author, Sussex material-physics lecturer Conor Boland, differentiated his lab’s work, which uses electromechanical sensing, from the Northwestern bandage, which uses electrochemical sensing, but said both approaches can have legitimate uses in human health care. For example, he said, his team is already working on turning the algae mixture into a material that mimics human skin’s mechanical properties, but also has the electronic capabilities to monitor blood pressure and breathing rate.
One particularly interesting future application may be pulse oximetry, the detection of oxygen saturation in the blood. Health researchers have documented a persistent problem with many current commercially available oximeters, which use optical sensors—they often measure oxygen saturation on people with darker pigment inaccurately.
“Ours is a physical measurement, you are putting it onto the skin and it’s measuring the artery pushing up against the skin,” Boland said. “It will always have the same signal as long as the material is calibrated.”
Boland concedes that the ecosystem around fully biocompatible and biodegradable devices is still in its infancy. While his lab’s device is fully degradable, any protective casing meant to extend its lifespan—he said the lab prototype is stable for about five or six hours—would not be.
“I would hope our work can show there is merit for the commercialization of such materials and open a broader range of research,” he said.
- Biodegradable Power Generators Could Power Medical Implants ›
- The Internet of Disposable Things Will Be Made of Paper and Plastic Sensors ›