Can Flow Batteries Finally Beat Lithium?
Nanoparticles may boost energy density enough for EVs

As she drives her electric vehicle to her mother’s house, Monique’s battery gauge indicates that it’s time to reenergize. She stops at a charging station, taps her credit card at the pump, inserts a nozzle into the car, and in 5 minutes exchanges 400 liters of spent nanofluid for fresher stuff. As she waits, a tanker pulls up to refill the station itself by exchanging tens of thousands of liters of charged for spent fuel. Monique closes her EV’s fueling port and heads onto the highway with enough stored energy to drive 640 kilometers (400 miles).
The battery in her EV is a variation on the flow battery, a design in which spent electrolyte can be replaced, the fastest option, or the battery could be directly recharged, though that takes longer. Flow batteries are safe, stable, long-lasting, and easily refilled, qualities that suit them well for balancing the grid, providing uninterrupted power, and backing up sources of electricity.
This battery, though, uses a completely new kind of fluid, called a nanoelectrofuel. Compared to a traditional flow battery of comparable size, it can store 15 to 25 times as much energy, allowing for a battery system small enough for use in an electric vehicle and energy-dense enough to provide the range and the speedy refill of a gasoline-powered vehicle. It’s the hoped-for civilian spin-off of a project that the Strategic Technology Office of the U.S. Defense Advanced Research Projects Agency (DARPA) is pursuing as part of a drive to ease the military’s deployment of all-electric supply vehicles by 2030 and of EV tactical vehicles by 2050.
Using lithium-based batteries would create its own set of problems. You’d need a charging infrastructure, which for the U.S. military would mean deploying one, often in inhospitable places. Then there’s the long charging time; the danger of thermal runaway—that is, fires; the relatively short working life of lithium batteries; and the difficulties of acquiring battery materials and recycling them when the old batteries are no longer any good. A battery that mitigates these problems is DARPA’s objective. The new flow battery seems to hit every mark. If it works, the benefits to the electrification of transportation would be huge.
Flow batteries are safe and long-lived
Nanoelectrofuel batteries are a new take on the reduction-oxidation (redox) flow battery, which was first proposed nearly a century and a half ago. The design returned to life in the mid-20th century, was developed for possible use on a moon base, and was further improved for use in grid storage.
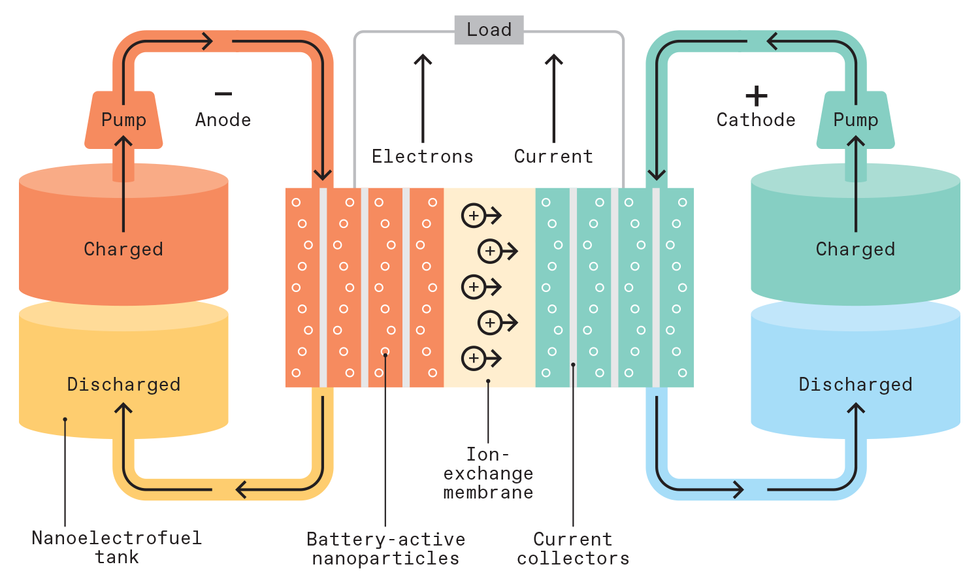
The cell of a flow battery uses two chemical solutions containing ions, one acting as the anolyte (adjacent to the anode), the other as the catholyte (near the cathode). An electrochemical reaction between the two solutions pushes electrons through a circuit. Typical redox flow batteries use ions based on iron chromium or vanadium chemistries; the latter takes advantage of vanadium’s four distinct ionic states.
On the chemical side of the reaction, each solution is continuously pumped into separate sides of a battery cell. Ions pass from one solution to the other by crossing a membrane, which keeps the solutions apart. On the electrical side, current moves from one electrode into an external circuit, circling around before returning to the opposite electrode. The battery can be recharged in two ways: The two solutions can be charged in place by a current moving in the opposite direction, the way conventional batteries are charged, or the spent solutions can be replaced with charged ones.
Besides beating lithium batteries in performance and safety, flow batteries also scale up more easily: If you want to store more energy, just increase the size of the solution storage tanks or the concentration of the solutions. If you want to provide more power, just stack more cells on top of one another or add new stacks.
This scalability makes flow batteries suitable for applications that require as much as 100 megawatts, says Kara Rodby, a technical principal at Volta Energy Technologies, in Naperville, Ill., and an expert in flow batteries. An example, she says, is the task of balancing energy flows in the power grid.
However, conventional flow batteries pack very little energy into a given volume and mass. Their energy density is as little as 10 percent that of lithium-ion batteries. It has to do with the amount of material an aqueous solution can hold, Rodby explains. There is only so much salt you can dissolve in a glass of water.
Therefore, flow batteries have so far been too bulky for most applications. To shrink them enough to fit in electric vehicles, you need to raise their energy density to that of lithium-ion batteries.

Nanoparticles boost flow battery’s energy density
One good way to add capacity to a flow battery is with nanofluids, which hold nanoparticles in suspension. These particles undergo redox reactions at the electrode surface similar to how the dissolved ions react in conventional flow batteries, but the nanofluids are more energy dense. Importantly, the nanofluids are engineered to remain suspended indefinitely, unlike other suspensions—for instance, sand in water. That indefinite suspension helps the particles move through the system and make contact with the electrodes. The particles can compose up to 80 percent of the liquid’s weight while leaving it no more viscous than motor oil.
Nanofluids suspended in water-based electrolytes were first investigated for this application in 2009 by researchers at Argonne National Laboratory and the Illinois Institute of Technology. The scientists found the nanofluids could be used in a system with an energy-storing potential approaching that of a lithium-ion battery and with the pumpable recharging of a flow battery. What’s more, the nanoscale particles could be made from readily available, inexpensive minerals, such as ferric oxide and gamma manganese dioxide for the anode and cathode materials, respectively.
Additionally, because the nanoelectrofuel is an aqueous suspension, it did not catch fire or explode, nor would the material be hazardous if the battery were to leak. The battery possessed a wide operational range of between -40 °C and 80 °C.
In 2013, the team received a three-year, US $3.44 million grant from the U.S. Department of Energy’s Advanced Research Projects Agency–Energy (ARPA-E) to build a prototype 1 kilowatt-hour nanoelectrofuel battery. The prototype’s success encouraged several of the principal investigators to spin off a company, called Influit Energy, to commercialize the technology. Through additional government contracts, the startup has continued to improve the components of the technology—the nanoelectrofuel itself, the battery architecture, and the recharging and delivery system.
John Katsoudas, a founder and chief executive of Influit, emphasizes the distinction between his company’s design and a conventional flow battery. “Our novelty is in doing what others have already done [with flow batteries] but doing it with nanofluids,” he says.
With the basic science problem resolved, Katsoudas adds, Influit is now developing a battery with an energy density rated at 550 to 850 watt-hours per kilogram or higher, as compared to 200 to 350 Wh/kg for a standard EV lithium-ion battery. The company expects larger versions would also beat old-style flow batteries at backing up the grid because the nanoelectrofuel can be reused at least as many times as a flow battery—10,000 or more cycles—and it will probably be cheaper.


The fuel would be created as needed, he says, eventually at such a scale as to replace fossil fuels. The fuel could be transported to depots much as gasoline is today, either by tanker trucks or via existing upgraded pipelines. At the depot, the spent fuel could be recharged with electricity from any source—solar, wind, hydroelectric, nuclear, or fossil fuels. The recharging could also be done at a service station or in the EV itself. In the latter case, the recharging would work just as it does for today’s battery electric vehicles.
What if there were a tanker crash or a pipeline rupture?
“The NEF turns into a pastelike substance, which you then sweep up,” Katsoudas explains. If you don’t want to wait for it to dry, he adds, you can add more water to reduce the acidity, “then you just shop-vac it up.”
What you do not want to do is throw that stuff away. “What is collected is the most valuable part of the battery,” he says. “There are processes to easily reconstitute the active material into a new nanofluid that can be reused.”
And what of the risks of the charged liquids intermingling? Katsoudas says they have demonstrated that directly mixing the different existing anolyte and catholyte liquids poses minimal safety hazards, and that since the next generation NEF materials will use identical liquids, the risks are minimized altogether.
Designing a flow battery for electric vehicles
On every count, nanoelectrofuel flow batteries appear to beat lithium-ion batteries for use in EVs and larger systems. Influit expects that its current generation of nanoelectrofuel, together with the entire ecosystem needed to produce, distribute, and recycle the fuel that the company is building around it, should cost $130/kWh when used in an EV. In comparison, lithium-ion batteries cost around $138/kWh. True, lithium-ion’s costs should drop below $100/kWh in a few years, but Influit expects its next-generation nanoelectrofuel to fall even further, to around $50 to $80/kWh. That next-gen system should have 5 times the energy density of present Li-ion systems.
Here’s what that means for an EV.
A typical EV battery today occupies about the same volume as would a flow battery with 400 liters of nanelectrofuel. If nanoparticles made up 30 percent of the weight of that fuel, the EV would have a range of only 105 km. Raise that to 40 percent, and the range would climb to 274 km. At 50 percent, it hits 362 km. And at 80 percent, it’s 724 km (450 miles). And that’s all assuming the flow battery’s tank remains the same size.
Influit has already achieved the 50 percent mark and has demonstrated an 80 percent nanoelectrofuel, says Aaron Kofford, a program manager in DARPA’s Strategic Technology Office.
For the military, nanoelectrofuel batteries have obvious advantages over lithium-ion batteries as well as internal combustion engines, Kofford says. In military fighting vehicles, protecting a vehicle’s fossil-fuel tank is critical, but that added protection weighs a lot and requires that the vehicle have a heavier suspension. That weight, in turn, reduces range and payload. Lithium-ion batteries, which are heavy in themselves and prone to fires, would also need to be heavily shielded against a shell hit.
By contrast, nanoelectrofuel batteries are fireproof, so the weight and safety issues are reduced tremendously. This video shows flammability tests with nanoelectrofuel samples:
Influit Energy
“At the system level, if we can take a chemistry that is inherently safe, we don’t need as much inner packaging in the battery itself,” Kofford says. They also don’t give off as much heat, so the vehicles are harder to spot from a distance, he adds. Civilian applications for nanoelectrofuel flow batteries beckon, notably in aviation. The reduced need for fire-safety systems in electric aircraft is a draw, observes Starr Ginn, NASA’s advanced air-mobility lead strategist.
With nanoelectrofuels, Ginn says, “You don’t [need] high-powered cables, you don’t have electromagnetic interference problems.” Nanoelectrofuel “just keeps checking these boxes off of all the things that are making it hard to build electric airplanes.”
Similarly, the U.S. Air Force Research Laboratory’s Transformational Capabilities Office is assessing how nanoelectrofuels could help in combat operations. Influit is also working with a commercial partner to pilot nanoelectrofuel flow batteries in their line of electric utility vehicles.
Lithium-ion batteries have a considerable head start
Considerably more work must be done to fulfill the potential of nanoelectrofuel technology. Influit and its government sponsors expect it will take two more years to put together all the pieces of a closed-loop system and to prove its value and scalability in a variety of applications. Katsoudas says that by 2025 or 2026, the world will be ready to give a serious look to nanoelectrofuel flow batteries for powering zero-emission vehicles, grid backup, electric utility vehicles, and the like.
Two possible barriers block the technology’s ascent: market forces and competing technologies.
Lithium-ion batteries are a mature technology and have a developed market. Hundreds of billions of dollars are being poured into the development and improvement of lithium batteries of all types, with governments underwriting much of the investment. For instance, DARPA, the Department of Energy, and the National Science Foundation are working with a host of companies to overcome the limitations of lithium batteries. Government research organizations in the EU, South Korea, and elsewhere are funding similar research. To overtake lithium technology, Influit will have to convince someone with extremely deep pockets to help it scale up—perhaps from its own logistics supply chain or from EV manufacturers.

Then there is the technological competition. News arrives nearly daily of yet another lithium-ion advance. One report from researchers at the Chinese Academy of Sciences boasts of a 711 Wh/kg lithium-ion battery. A Chinese manufacturer claims that a new lithium manganese iron phosphate battery chemistry will power an EV for 1,000 km on a single charge and last 130 years. Other announcements involve significant improvements in rapidly charging lithium-based batteries and making them safer for use in military vehicles.
Then there are the new battery chemistries that are not lithium based—for instance, sodium-ion and graphene-based batteries. And there have been advances in grid-scale batteries involving liquid metal technology, and improved traditional flow-battery technology using lithium sulfur.
Other direct competitors to Influit include e-fuels (synthetic carbon-based and carbon-neutral fuels produced from captured carbon dioxide and water using renewable electricity sources), as well as liquid organic hydrogen. Both fuels aim to directly displace fossil fuels. For Influit to gain market adoption, Volta’s Rodby says, the company will need to articulate what the “market differentiator” for nanoelectrofuels is. Right now it seems the technology is a particularly good fit for the Department of Defense, which would likely be willing to pay a premium for it. As the largest user of fossil fuels in government, the DOD alone may enable Influit Energy to get to scale.
Of course, it may turn out that nanoelectrofuels find a home in other applications, such as boats, trains, or planes. For example, the largest cargo container ship carries some 15 million liters of fuel. If some portion of that were nanoelectrofuel that could be continually recharged, it might be possible to reduce the ship’s carbon footprint.
There is a rich history of apparently superior technologies that came too late or too early to displace the incumbents. Nanoelectrofuel flow batteries appear to be superior to what we have today. Perhaps they will also be lucky.
This article appears in the February 2024 print issue.


