Argonne National Lab Issues Lithium-Sulfur Battery Research Update
While lithium-ion batteries are the mainstay of electric vehicles today, they do have certain drawbacks. They tend to overheat and they don’t always last as long as we might like. In addition, some of the raw materials are in short supply, which means the cost to manufacture them is rising rather than falling as expected due to economies of scale.
Scientists at the U.S. Department of Energy’s Argonne National Laboratory are researching solutions to these issues by testing new materials in battery construction. One such material is sulfur, which is abundant and cost effective. It can also store significantly more energy than traditional lithium-ion batteries. The researchers claim 2600 Wh per kilogram is possible.
In a recent study published in the journal Nature Communications, the researchers reported on advances in sulfur-based battery research achieved by creating a layer within the battery that adds energy storage capacity while nearly eliminating a traditional problem with sulfur batteries that caused corrosion. “These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development. We’re one step closer to seeing this technology in our everyday lives,” says Wenqian Xu, who is a member of the research team.
The promising new battery design pairs a sulfur-infused positive electrode with a lithium-metal negative electrode. In between those components is the electrolyte — the substance that allows ions to pass between the two ends of the battery. Early lithium-sulfur batteries did not perform well because polysulfides dissolved into the electrolyte, causing corrosion. This polysulfide shuttling effect negatively impacts battery life and lowers the number of times the battery can be recharged.
To prevent this polysulfide shuttling, the researchers tried placing a redox-inactive interlayer between the cathode and anode. The term “redox-inactive” means the material does not undergo reactions like those in an electrode. But this protective interlayer is heavy and dense, reducing energy storage capacity per unit weight for the battery. It also does not adequately reduce shuttling, which has proven to be a major barrier to the commercialization of lithium-sulfur batteries.
To address this, researchers developed and tested a porous sulfur-infused interlayer. Tests in the laboratory showed initial capacity about three times higher in lithium-sulfur cells with this active — as opposed to inactive — interlayer. The cells with the active inter ayer maintained high capacity over 700 charge/discharge cycles.
“Previous experiments with cells having the redox-inactive layer only suppressed the shuttling, but in doing so, they sacrificed the energy for a given cell weight because the layer added extra weight,” says Guiliang Xu, an Argonne chemist and co-author of the paper. “By contrast, our redox-active layer adds to energy storage capacity and suppresses the shuttle effect.”
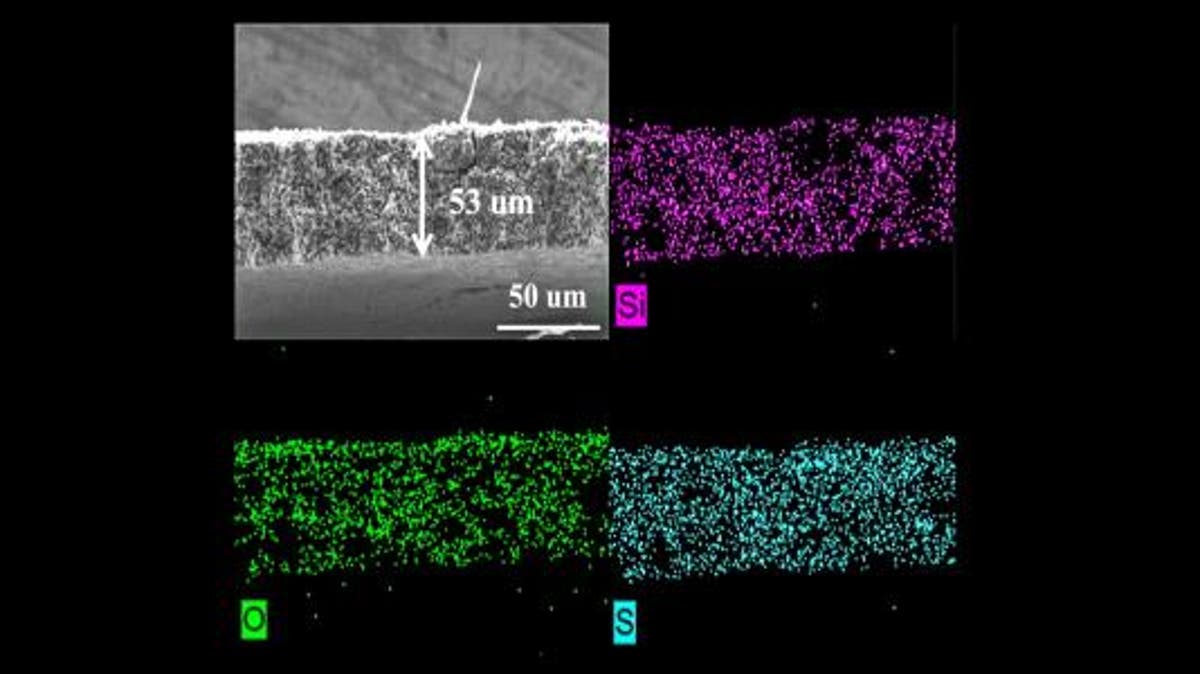
To further study the redox-active layer, the team conducted experiments at Argonne’s Advanced Photon Source, a DOE Office of Science user facility. The data gathered from exposing cells with this layer to X-ray beams allowed the team to ascertain the inter layer’s benefits.
The data confirmed that a redox-active interlayer can reduce shuttling, reduce detrimental reactions within the battery, and increase the battery’s capacity to hold more charge and last for more cycles. “These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development,” says Wenqian Xu, “We’re one step closer to seeing this technology in our everyday lives.” Going forward, the team wants to evaluate the growth potential of the redox-active inter layer technology. “We want to try to make it much thinner, much lighter,” Guiliang Xu says,
In summary, the researchers write, “We propose a polar and redox-active interlayer concept for high-energy and long-cycling Li-S batteries, in which sulfur is embedded into a polar platelet ordered mesoporous silica to form an interlayer. Interestingly, sulfur storage/trapping occur at the polar silica while electron transfer at conducting agent in pOMS/Sx IL during charge-discharge.
“During the electrochemical processes, this interlayer not only fulfils the role of effectively preventing the shuttling of long-chain polysulfides, but also contributes to enhance the areal capacity to the cell. The cell with optimal interlayer delivers an areal capacity of >10 mAh cm−2 with the benefit of high sulfur loading of >10 mg cm−2 and stable cyclability for 700 cycles, even under high specific current cycling and low electrolyte/sulfur ratio. These attributes can increase the practical specific energy of Li-S batteries.”
 Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
About Argonne National Laboratory
Argonne is America’s first national laboratory. It seeks solutions to pressing national problems in science and technology and conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership, and prepare the nation for a better future.
One of its preeminent resources is the Advanced Photon Source facility which provides high-brightness X-ray beams to a diverse community of researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. These X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems.
This includes the insertion devices that produce extreme-brightness X-rays prized by researchers, lenses that focus the X-rays down to a few nanometers, instrumentation that maximizes the way the X-rays interact with samples being studied, and software that gathers and manages the massive quantity of data resulting from discovery research at the APS, which was instrumental in supporting the lithium-sulfur battery research described above.
Lithium-Sulfur Research Around The World
Many battery researchers are exploring the feasibility of sulfur batteries, primarily because sulfur is one of the most abundant and readily available materials on Earth. While lithium-ion batteries have performed amazingly well in getting the EV revolution started, they are just a precursor to the batteries that await us in years to come.
Researchers in Australia are exploring batteries that combine sulfur with sodium. Theionm, a German battery startup is also pursuing lithium-sulfur research amid claims such batteries could triple the range of electric vehicles. A year ago, we reported on research conducted at the University of Michigan into lithium-sulfur batteries that are said to last for up to 1000 charge/discharge cycles.
The problem, as always, is getting advanced battery technology out of the lab and into commercial production. It was five years ago that we reported on research at MIT that promised lithium-sulfur batteries for grid-scale storage applications. To our knowledge, no commercial applications have resulted from that research as of yet. The fascinating part of the MIT studies was the claim that those batteries would cost only 1% of conventional lithium-ion batteries. Can you imagine how that would disrupt the utility industry!
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica.TV Video

CleanTechnica uses affiliate links. See our policy here.