Dr Christine Joblin of the CNRS and her colleague Dr Hassan Sabbah of the University of Toulouse III – Paul Sabatier highlight the ability of the AROMA molecular analyser to understand the origin of large carbonaceous molecules in cosmic environments.
The James Webb Space Telescope (JWST, NASA/ESA/CSA) has just revealed its amazing capabilities to study the Universe at infrared wavelengths. Among the data, we expect unprecedented information about large carbonaceous molecules that are key species in star- and planet-forming regions.2 These molecules are dominated by the family of polycyclic aromatic hydrocarbons (PAHs) – well-known terrestrial pollutants that are major by-products of combustion processes. It remains to be understood why these PAHs are so abundant in astrophysical environments (they typically contain 10% of the carbon).
What are the mechanisms that recycle carbon in space and (preferentially) form these molecules? So far, the research has been mainly guided by our knowledge of flame chemistry and the proposal that astro-PAHs are produced at the end of the life of carbon-rich stars, in hot and dense envelopes in which the formation of dust and molecular species, such as PAHs, is favoured. Still, as of today, we have no clear observational diagnosis to demonstrate this scenario. In addition, the individual PAHs that have been identified so far (indene C9H8 and cyano-naphthalene C10H7CN)4,5 are present in the TMC-1 dark molecular cloud and are most likely products of very low temperature gas-phase chemistry.
The PAH model
Over the past decade, the unambiguous detection of C60 buckminsterfullerene and its C60+ cation in a variety of astrophysical environments has been a major achievement. It has allowed support for the PAH model. This model, proposed in the 1980s, explains that all the strong aromatic infrared bands observed in emission come from radiative cooling of PAHs heated by ultraviolet photon absorption. We now know that other carriers, such as fullerenes, must be included. Compared to PAHs, the formation of fullerenes requires more extreme conditions – in particular, higher temperatures. This issue motivates studies to propose formation scenarios for C60 in astrophysical environments. These include processing of grains by heat, shocks or high-energy ions and UV photo-processing of large PAHs.6,7
In this paper, we present our approach to address the question of the formation of astro-PAHs and fullerenes from an experimental point of view. This approach is based on two pillars. The first pillar is to use a variety of reactors to produce samples that can be considered as laboratory analogues of stardust. Although it is impossible to mimic the physical and chemical conditions of dying stars, a new machine, Stardust,8 has been built in Madrid (ICMM/CSIC) to advance in this field by offering features not available in other experimental setups, such as the controlled production of (e.g, carbon, silicon) atoms to drive the chemistry.
Cold plasmas are also used as an alternative method to study the impact of complexity on chemistry. In particular, we study the role of metal (e.g., iron) atoms in the formation of dust and large carbonaceous molecules with our colleagues from LAPLACE (CNRS/ Université Toulouse III-Paul Sabatier).9 The second pillar consists of studying samples of extra-terrestrial matter (meteorites) that are rich in carbon. This is the case of carbonaceous chondrites like Murchison and Allende but also of the polymict ureilite meteorite Almahata Sitta (AhS). These two pillars share the common interest of probing the carbonaceous molecular content of samples that may be in the form of dusty deposits or bulk materials, as illustrated in Fig. 1. This topic motivated the development of the Astrochemistry Research of Organics with Molecular Analyzer (AROMA) setup.
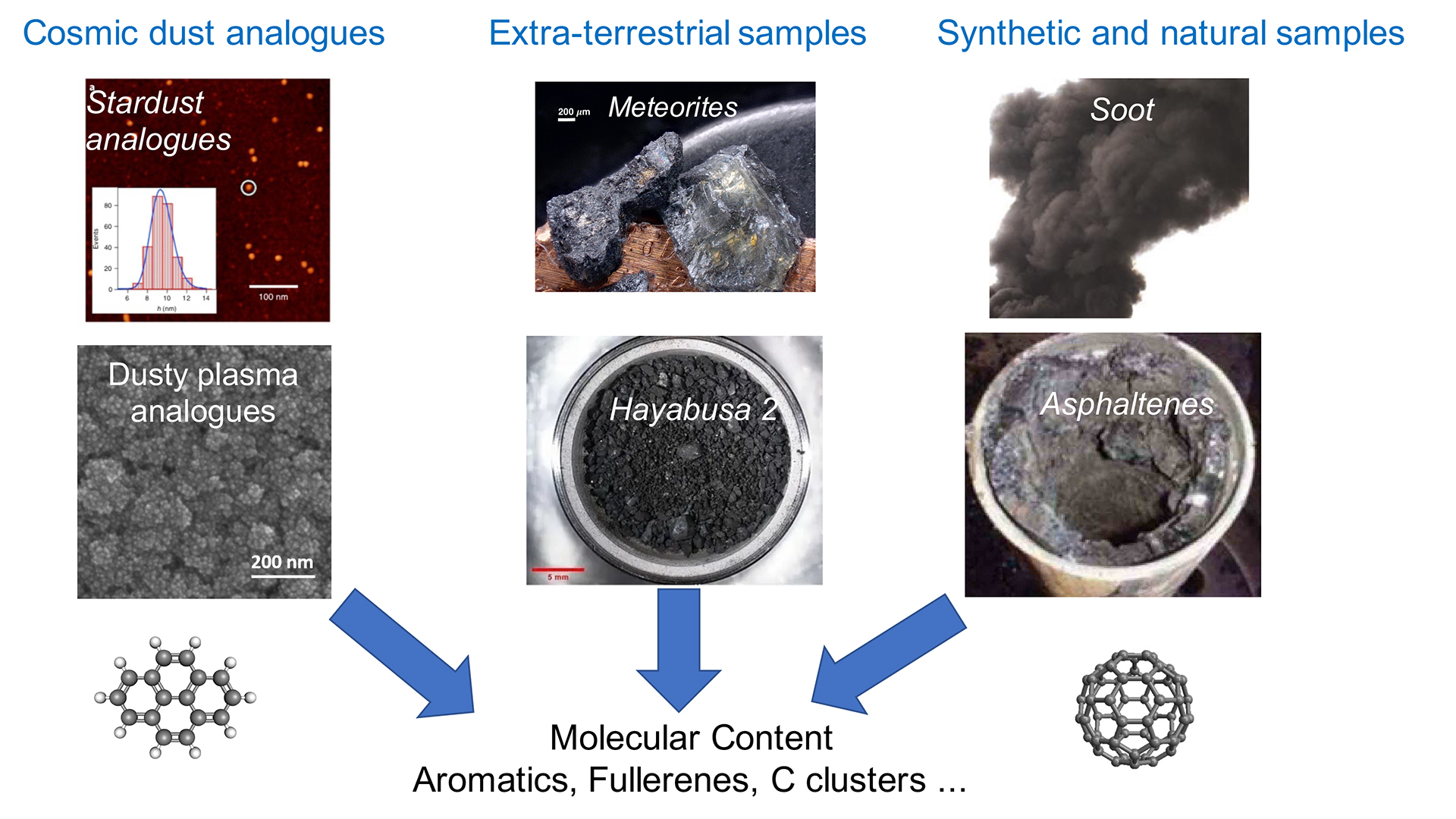
AROMA: the molecular analyzer
The AROMA setup10 has been developed in the framework of the Nanocosmos ERC Synergy project.1 The construction was done by Fasmatech, a young Greek company, following the requirements of our scientific and engineering team. The main objective is to trace the molecular content of various solid samples, especially in large molecules such as polycyclic aromatic hydrocarbons, carbon clusters and fullerenes.
AROMA, as shown in Fig. 2a, consists of three main components: the ion source, the ion trap, and the mass analyzer.
In the ion source, millimetre-sized samples are positioned on an XY manipulator and subjected to laser desorption/ionisation (LDI) techniques. LDI techniques, using a single laser step, are powerful tools for directly probing non-volatile organic species in native or artificial matrices without the need for tedious extraction procedures. In the case of high molecular weight compounds, such as biomolecules, a matrix-assisted LDI (MALDI) technique is typically used. The specificity of AROMA is the use of the L2MS technique, in which the desorption and ionisation processes are separated in time and space and performed with two different lasers. A pulsed infrared laser is used to desorb neutral molecules, followed after a few microseconds by a pulsed ultraviolet laser to selectively ionise the molecules of interest.13 L2MS offers a much higher sensitivity than one-step LDI to the targeted molecules (e.g., in our case, PAHs and fullerenes). In addition, AROMA offers the possibility to isolate and trap ions of a specific mass and to study their fragmentation by collisions with a gas or by absorption of photons from a tuneable laser. Both techniques help us to elucidate the molecular structures of the dominant species. Finally, the ions are mass separated using a high-resolution time-of-flight mass spectrometer (104 resolving power).
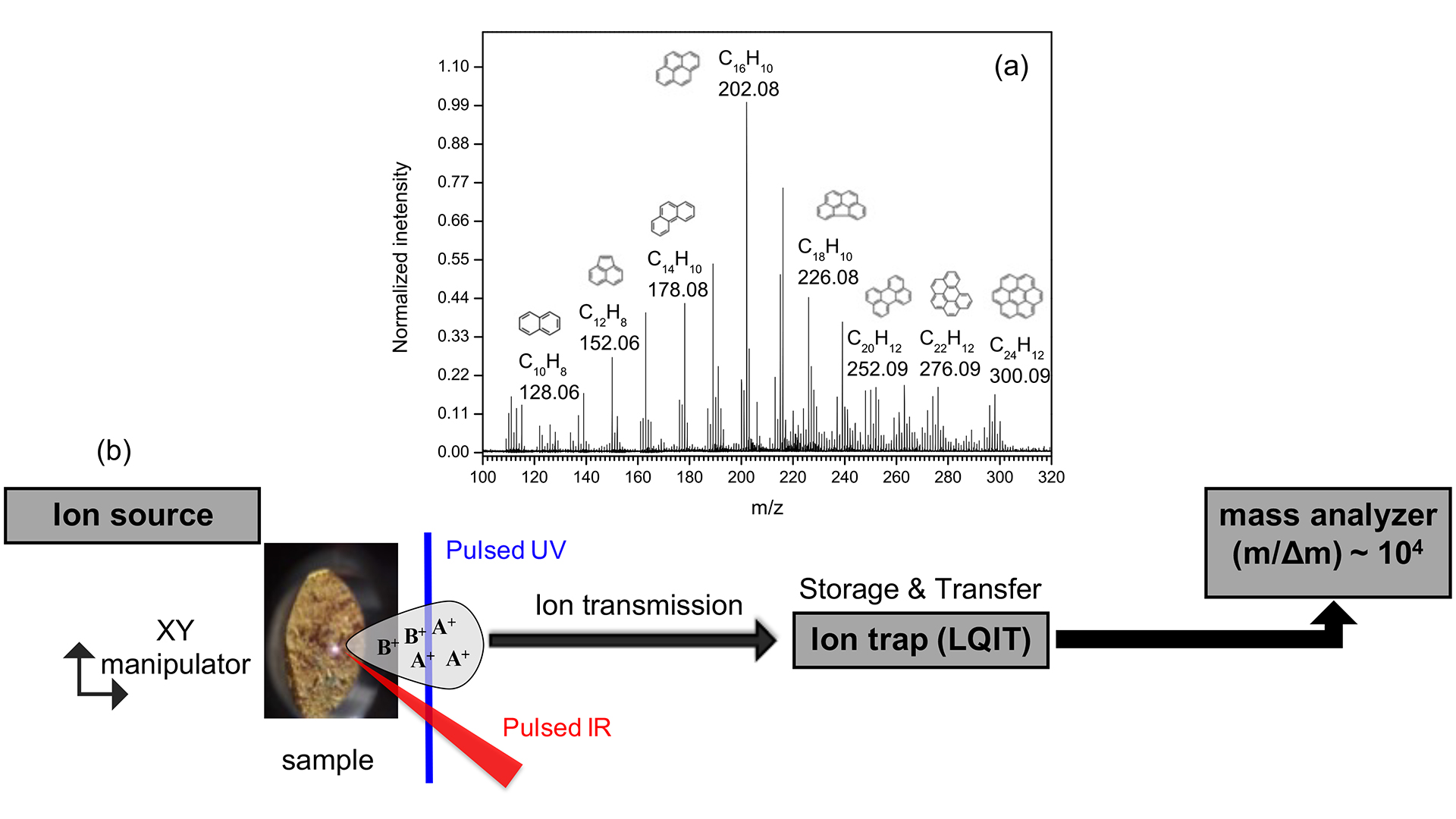
Fig. 2 shows an example of a mass spectrum recorded for a few mg of crushed powder from the Murchison meteorite. It plots the intensity of the ion signal as a function of the mass-to-charge ratio. This allows us to unequivocally assign a chemical formula to each detected peak and associate it with a pure carbon (Cx) or hydrocarbon (CxHY) species. Other elements, especially in atomic form, may also be present (for example, Na, Al, K, Fe, etc.). Our assignment accuracy approaches 0.01 at a mass/charge ratio m/z ~300. This is our limitation to assign with high confidence organic compounds with N, O and S atoms in their chemical formula. Eventually, hundreds of peaks are identified in the mass spectra with notable discrepancies between different samples. The latter can be used to trace the chemical history of each sample and are not a bias of our analysis.
For example, in the analysis of terrestrial soot samples,11 we were able to track the evolution of carbonaceous molecular families, as a function of height above the burner (Z) and demonstrate efficient thermal processing of the large PAH population to produce hydrogenated carbon clusters (HC clusters) and then fullerenes (see Fig. 3a). To recover this chemical information, large mass spectrometry datasets must be reduced to relevant parameters such as molecular families. For this, our methodology consists in calculating double bond-equivalent (DBE) values, which are representative of the unsaturation level of the molecules. The different ranges of DBE values allow us to disentangle the molecular families: PAHs, HC clusters, aliphatic species (very rare in our samples because the ultraviolet laser is not adapted to their ionisation), carbon clusters and fullerenes.
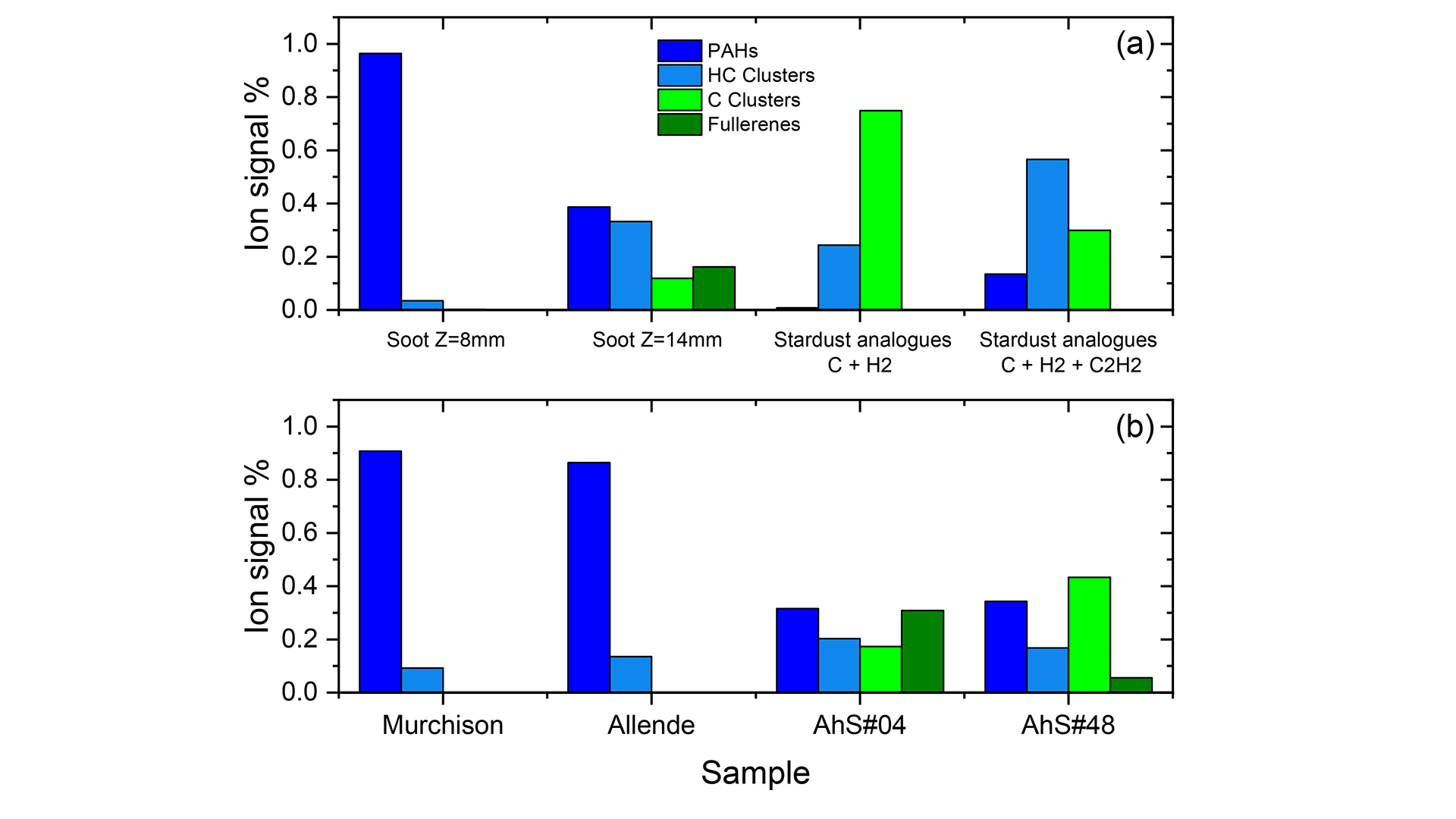
Fig. 3 illustrates the method and shows the results of molecular family analysis for several samples, including terrestrial soot, stardust analogues (Fig. 3a), and several meteorites (Fig. 3b). PAHs dominate the molecular composition of carbonaceous chondrites (Murchison and Allende). In contrast, the two AhS fragments, AhS#04 and AhS#48, show a greater diversity of molecular families, which have been shown to originate from different cosmic reservoirs.
All mass spectra associated with published work are publicly available in the AROMA database. The database provides the ability to calculate and plot DBE values, and to perform molecular family analyses. We are also developing a method to assess the similarity between recorded mass spectra and how it can be used to provide faster investigation and additional information about the chemical history of a sample.
Amazing results and perspectives
Initially seen as an analytical tool to support the Stardust machine, AROMA has now become a key facility to contribute to our understanding of the origin of large carbonaceous molecules in cosmic environments. In the term cosmic, we include astrophysical environments (e.g., evolved stars as stardust factories) but also our Solar System, via extra-terrestrial samples that we can access in the laboratory.
In connection with the Stardust machine, the most important result we have obtained so far is the near absence of aromatics formed in the chemistry of atomic carbon with molecular hydrogen. This casts doubt on the possibility of forming abundant PAHs by gas-phase chemistry in the envelopes of evolved stars.
But the result that opens up the greatest prospects is certainly the unexpected but firm detection of fullerenes, from C30 to at least C100, in the Almahata Sitta (AhS) polymict ureilite meteorite. We are now in a rather unique position to search for fullerenes in a variety of extra-terrestrial samples. In particular, we were recently fortunate to receive two grains from the asteroid Ryugu that were carried by JAXA’s Hayabusa2 sample return mission. Ryugu is a C-type asteroid that is considered one of the most pristine objects in the Solar System. The analysis of this type of object will allow us to circumvent the problem of meteorite alteration linked to the shocks during their ejection and to the heating during their entry into the Earth’s atmosphere, as well as to a possible terrestrial contamination. We believe that this work can give us new insight into the origin of fullerenes in astrophysical environments.
We also expect a big step forward with the next JWST results, including the identification of individual species among the molecular families studied by AROMA: fullerenes, PAHs, C clusters, as well as new information on the chemical relationship between these families and with the dust particles.
The prospect of changing our view of the cosmic carbon cycle has never been closer and it is quite exciting. In addition to the fantastic challenges that JWST and Hayabusa2 have met, innovation will also be required on the laboratory side. A major advance that we hope to address in our research activity is the possibility to couple the L2MS technique to an OrbitrapTM mass analyser that offers very high mass resolution and thus opens new perspectives to trace the origin of organic matter in samples of cosmic interest.
Acknowledgement
This research was supported over the period 2014-2021 by the ERC under the European Union’s Seventh Framework Programme ERC-2013-SyG, Grant Agreement no. 610256, Nanocosmos.
References (with open access)
1: Nanocosmos ERC Synergy project: J Cernicharo, C. Joblin, J.-A. Martín Gago, https://nanocosmos.iff.csic.es/
2: Early Release Science of JWST: “Radiative feedback from massive stars”: Berné, O, Habart, E, Peeters, E, et al., Publ. Astron. Soc. Pac. 134 (1035) (2022), id.054301; https://arxiv.org/abs/2201.05112
3: “Astro-PAHs from space missions to laboratory astrophysics”; Joblin, C, The Innovation Platform issue 8 (2021), p.228-231; https://www.innovationnewsnetwork.com/astro-pahs-space-missions-to-laboratory-astrophysics/15527/
4: Observation of indene in the dark molecular cloud TMC-1: Cernicharo, J, et al., Astron. & Astrophys. 649 (2021), id.L15, 12 pp.; https://doi.org/10.1051/0004-6361/202141156
5: Observation of cyanonaphthalene in the dark molecular cloud TMC-1: McGuire, B. A., et al., Science 371 (2021), 1265–1269; https://arxiv.org/pdf/2103.09984.pdf
6: Discussion about formation scenarios of C60 in planetary nebulae: Cami, J., et al., Galaxies 6 (4) (2018), p. 101; https://www.mdpi.com/2075-4434/6/4/101
7: Scenario to form C60 from large PAHs: Berné, O, Tielens, A G G M, Proc. Natl. Acad. Sci. U.S.A. 109 (2012), 401-406; https://doi.org/10.1073/pnas.111420710
8: Stardust machine and the (non)formation of aromatics in evolved stars: Martínez, L, Santoro, G, Merino, P, et al., Nature Astron. 4 (2020), 97-105; https://hal.archives-ouvertes.fr/hal-03085475
9: Role of metals in (star)dust chemistry investigated in cold plasmas: Bérard, R, et al., Front. astron. space sci. 8 (2021), 654879; https://hal.archives-ouvertes.fr/hal-03227179
10: AROMA setup: Sabbah, H, et al., Astrophys. J. 843 (2017), id. 34, 8 pp.; https://arxiv.org/ftp/arxiv/papers/1705/1705.09974.pdf
11: Analysis of soot samples with AROMA: Sabbah, H, et al., Proc Combust Inst 38 (2021), 1241–1248; https://doi.org/10.1016/j.proci.2020.09.022
12: Detection of fullerenes in the Almahata Sitta meteorite: Sabbah, H, et al., Astrophys. J. 931 (2022), id.91; https://iopscience.iop.org/article/10.3847/1538-4357/ac69dd
13: First presentation of the L2MS technique and its application to extra-terrestrial samples: Spencer, M K, Hammond, M R, and Zare, R N, Publ. Astron. Soc. Pac. 105 (2008), 18096-18101; https://doi.org/10.1073/pnas.0801860105
Please note, this article will also appear in the eleventh edition of our quarterly publication.









