Researchers Propose Non-Aqueous Manganese Metal Batteries
At CleanTechnica, we strive to keep our readers informed about new technologies that may signal better batteries for electric vehicles are on the horizon. This month, SK On reportedly is set to introduce two new LFP batteries to counteract concerns that EV drivers have. According to Bloomberg, the South Korean company will soon introduce its Winter Pro batteries that extend charging capacity by 16% and enhance energy density by 19% compared to traditional batteries at -20º C (-4º F). In addition, the company says its latest SF+ batteries can reach an 80% state of charge in just 15 minutes — 3 minutes faster than it regular SF batteries.
That’s all good news, but researchers in China say they may have found the key to manufacturing manganese batteries that have higher energy density and cost less than any lithium-based products. Manganese is far more abundant in the Earth’s crust, which means batteries that use it can cost less than lithium batteries.
Research into manganese batteries is not new, but until this point, their performance has been disappointing. What the researchers have discovered is that using a halogen infused non-aqueous electrolyte — specifically one that incorporates chlorine — makes manganese metal batteries possible that have long life and higher energy density without the dendrite issues that plague manganese batteries that use aqueous electrolytes. The research was published on March 20, 2024 in the journal Joule.
A Breakthrough In Manganese Batteries
Led by Wei Chen of the University of Science and Technology of China, the researchers developed a halogen-mediated non-aqueous electrolyte that prevents dendrites from forming on the anode. The technology is “perfectly compatible” with manganese and keeps the element from reacting with the electrolyte as it would an aqueous solution, they claim in the study. The manganese battery has a dissolution/deposition efficiency of more than 90% and “can cycle stably for over 1,000 h.”
In the introduction to their research, the team writes that manganese, which is widely available in the Earth’s crust at concentrations of 1000 ppm, is one of the essential elements in all known living organisms. In addition, unlike highly reactive alkaline earth metals like lithium, manganese is stable in air, which means it can be handled and stored in ambient condition, further lowering its production and storage costs. This gives it the lowest price among the frequently reported metal anodes. Aside from its low cost, it also provides the largest theoretical volumetric capacity based on its two electron transfer property and high density.
 Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
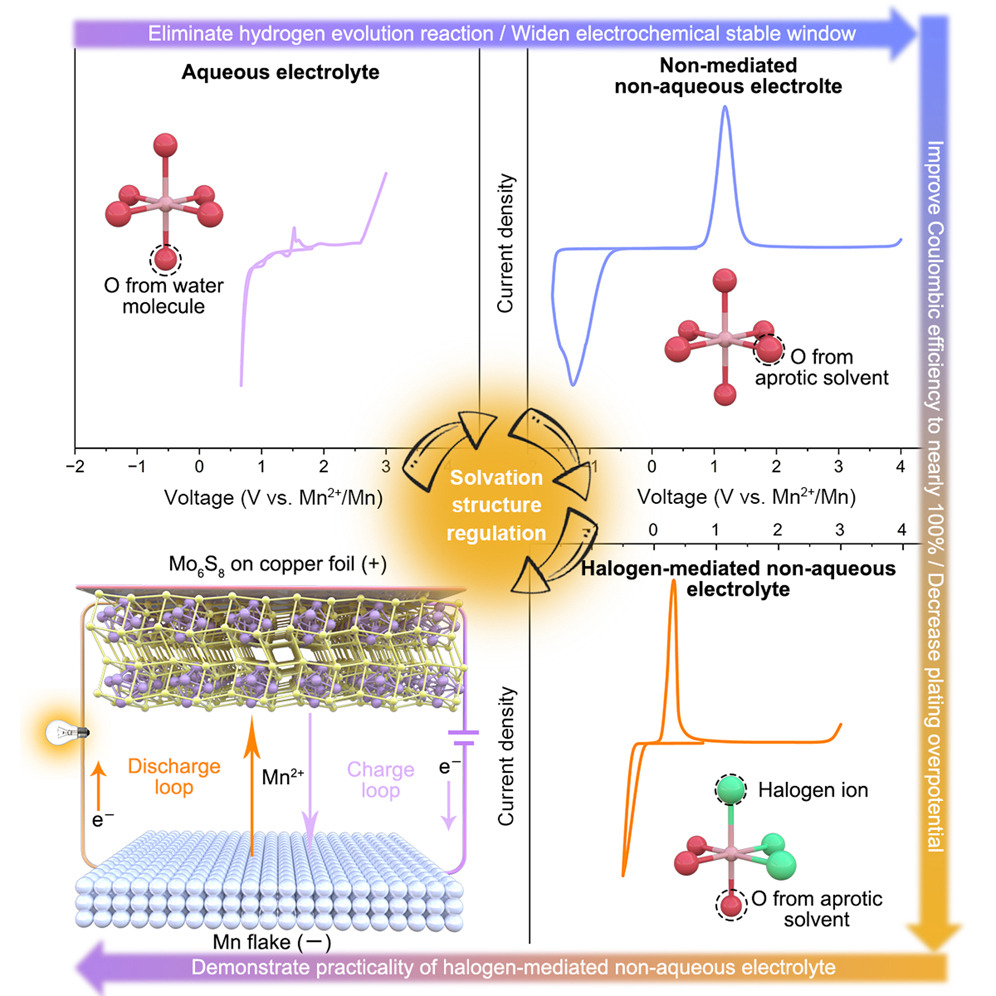
“Manganese ions … present a halogen-bridged cluster structure, which is the key to reducing the manganese deposition overpotential and avoiding side reactions, thus ensuring stable cycling of the battery,” the authors wrote. “It should be noted that as [the electrolyte] has greatly decreased manganese plating overpotential, this halogen-mediated strategy can also trigger inspirations for metal electroplating industry to increase the energy efficiency.” The research found that a halogen-mediated non-aqueous electrolyte is perfectly compatible with manganese metal, and the severe dendrite problem in aqueous electrolytes is eradicated.
In the jargon of the scientific world, the researchers note they have developed a halogen modulated non-aqueous electrolyte for manganese metal batteries that have a highly reversible, stable, and dendrite-free manganese anode. Combining theoretical calculations and dynamic simulations with experimental measurements, active cationic species in the experimental batteries were revealed to be [Mn2(μ-Cl)3(THF)6]+, where three bridged Cl atoms play a critical role in decreasing the manganese plating and stripping over-potential and increasing the Coulombic efficiency. Furthermore, it was demonstrated that the manganese batteries were both chemically and electrochemically stable.
The Takeaway
This research points the way toward batteries that are significantly more energy dense than any lithium-ion batteries currently available, while being much cheaper to produce thanks to the relative abundance of manganese. Although lithium prices have declined dramatically over the past 12 months, lithium is still far more costly at $1,250 per ton for spodumene, the ore that is the source for the lithium used in batteries. Manganese ore is priced at around $5.00 per ton. That makes it abundantly clear why this research could be big news for energy storage of all kinds in the future.
Of course, this is all happening in a laboratory at present. It’s a long and winding road from the lab to commercial production, as anyone who has been following the progress of QuantumScape and StoreDot for the past five years. If any of us are still alive in 2030, we may be driving inexpensive electric cars with manganese batteries, assuming international conflicts and trade wars don’t get in the way.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica.TV Video

CleanTechnica uses affiliate links. See our policy here.