UPDATE 4 APRIL 2024: In 2021, when IEEE Spectrum published the article that follows, there was optimism that the addition of graphene to sodium-ion anodes could not only narrow the gap in energy density between sodium-ion batteries and their lithium-ion counterparts, but that it might also yield enough of a performance boost for battery chemistries starring sodium to bring lithium’s days as the preferred element for energy storage to an end. But by July 2023, most sodium proponents were accepting the wisdom of the adage “If you can’t beat ‘em, join ‘em.” Adding a dash of sodium to lithium-ion batteries, researchers at Arizona State University found, would lower the lithium batteries’ cost without significantly diminishing performance. By that October, the DRX Consortium, led by Lawrence Berkeley National Laboratory, had announced plans for incorporating DRX, short for disordered rock salt, into lithium-ion batteries. The addition, they said, would triple those batteries’ energy density while still lowering their cost.
Still, some advocates holding out hope for a complete shift from lithium to sodium were undeterred. CATL, the world’s biggest lithium-ion battery manufacturer, is still working toward making sodium chemistry commercially viable. Its second-generation sodium-ion batteries, says company founder and chairman Robin Zeng, will allow reasonably priced cars to go up to 500 kilometers on a single charge. —IEEE Spectrum
Original story from 11 September 2021 follows:
After years of anticipation, sodium-ion batteries are starting to deliver on their promise for energy storage. But so far, their commercialization is limited to large-scale uses such as storing energy on the grid. Sodium-ion batteries just don’t have the oomph needed for EVs and laptops. At about 285 Wh/kg, lithium-ion batteries have twice the energy density of sodium, making them more suitable for those portable applications.
Researchers now report a new type of graphene electrode that could boost the storage capacity of sodium batteries to rival lithium’s. The material can pack nearly as many sodium ions by volume as a conventional graphite electrode does lithium. It opens up a path to making low-cost, compact sodium batteries practical.
Abundant and cheap, and with similar chemical properties as lithium, sodium is a promising replacement for lithium in next-generation batteries. The stability and safety of sodium batteries makes them especially promising for electronics and cars, where overheated lithium-ion batteries have sometimes proven hazardous.
“But currently the major problem with sodium-ion batteries is that we don’t have a suitable anode material,” says Jinhua Sun, a researcher in the department of industrial and materials science at Chalmers University of Technology.
For the battery to charge quickly and store a lot of energy, ions need to easily slip in and out of the anode material. Sodium-ion batteries use cathodes made of sodium metal oxides, while their anodes are typically carbon-based anodes just like their lithium cousins; although Santa Clara, California-based Natron Energy is making both its anodes and cathodes out of Prussian Blue pigment used in dyes and paints.
Some sodium battery developers are using activated carbon for the anode, which holds sodium ions in its pores. “But you need to use high-grade activated carbon, which is very expensive and not easy to produce,” Sun says.
Graphite, which is the anode material in lithium-ion batteries, is a lower cost option. However, sodium ions do not move efficiently between the stack of graphene sheets that make up graphite. Researchers used to think this was because sodium ions are bigger than lithium ions, but turns out even-bigger potassium ions can move in and out easily in graphite, Sun says. “Now we think it’s the surface chemistry of graphene layers and the electronic structure that cannot accommodate sodium ions.”
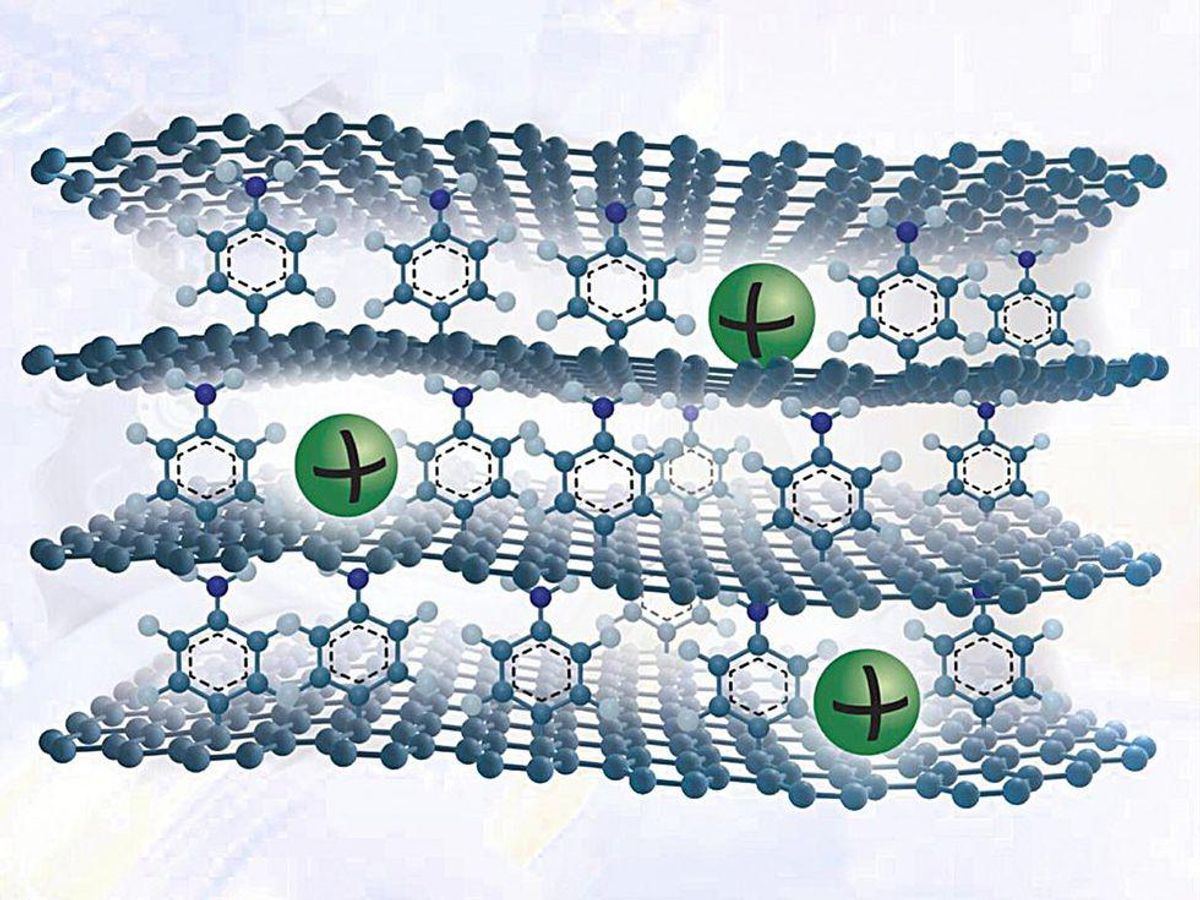
He and his colleagues have come up with a new graphite-like material that overcomes these issues. To make it, they grow a single sheet of graphene on copper foil and attach a single layer of benzene molecules to its top surface. They grow many such graphene sheets and stack them to make a layer cake of graphene held apart by benzene molecules.
The benzene layer increases the spacing between the layers to allow sodium ions to enter and exit easily. They also create defects on the graphene surface that as as active reaction sites to adsorb the ions. Plus, benzene has chemical groups that bind strongly with sodium ions.
This seemingly simple strategy boosts the material’s sodium ion-storing capacity drastically. The researchers’ calculations show that the capacity matches that of graphite’s capacity for lithium. Graphite’s capacity for sodium ions is typically about 35 milliAmpere-hours per gram, but the new material can hold over 330 mAh/g, about the same as graphite’s lithium-storing capacity.
- Graphene Composite Offers Critical Fix for Sodium-ion Batteries ... ›
- Here's a Peek at the First Sodium-ion Rechargeable Battery - IEEE ... ›
- Sodium-Ion Batteries Poised to Pick Off Large-Scale Lithium-Ion ... ›
- Graphene Gives 3D Graphite Twisted Powers - IEEE Spectrum ›
Prachi Patel is a freelance journalist based in Pittsburgh. She writes about energy, biotechnology, materials science, nanotechnology, and computing.